Physics of somite formation
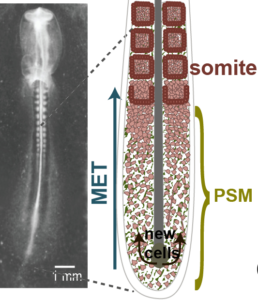
Tissue patterning is a critical step in embryonic development as it defines the boundaries between various tissues from which organs emerge. Boundary formation entails complex biochemical signaling that functions in concert with mechanical cues to generate distinct and robust structure of the right size, at the right place.
One such striking example is segmentation and shaping of somites during the early stages of vertebrate embryonic development. Somites are multi-cellular structures that pinch-off periodically at the anterior side of the mesodermal tissue of the tail, as it undergoes mesenchymal to epithelial transformation. Little is known about the parameters leading to the mechanical instabilities at the tissue level, leading to the formation go somites. We use in vivo and ex vivo approaches, combining biophysics, soft matter, and imaging techniques, to study the mechanical instability during somitogenesis in chicken embryos and try to pin point the cellular actors involved in this remarkable process.
Motivated applicants are encouraged to apply. We have several job openings.
Axial elongation in chicken embryos
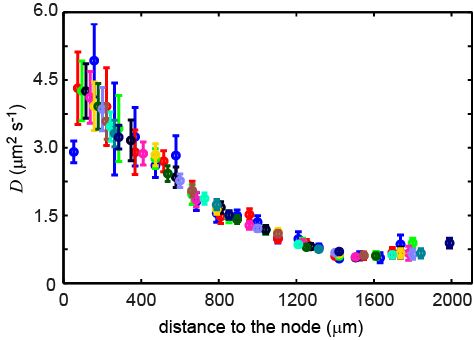
In this project, we study cell dynamics and mesodermal mechanics during the axis elongation process in chicken embryo. By combining experiments with cell tracking and physical modelling, we show that cells in the paraxial mesoderm move randomly. We can define an effective diffusion for these cells originating from their activity that is coupled to the morphogen gradients along the axis. We show that treating the mesoderm as an active fluid with proper boundary condition can account for the observed axial elongation. The fluid behavior of the paraxial mesoderm was established through rounding and micropipette aspiration experiments. In parallel, we show that this elongation is autonomous to the paraxial mesoderm, as explants of the mesoderm elongate in groove shaped channels.
- A. Michaut, A. Mongera, A. Gupta, M. Serra, P. Rigoni, J. G. Lee, F. Duarte, A. R. Hall, L. Mahadevan*, K. Guevorkian*, O. Pourquié*, “Activity-driven extracellular volume expansion drives vertebrate axis elongation“, bioRxiv (*co-corresponding author).
- I. Regev*, K. Guevorkian*, O. Pourquié, and L. Mahadevan, “Motility-gradient induced elongation of the vertebrate embryo”, bioRxiv 187443. (*equal contribution).
- M. Oginuma, P. Moncuquet, F. Xiong, E. Karoly, J. Chal, K. Guevorkian, and O. Pourquié, “A gradient of glycolytic activity coordinates FGF and Wnt signaling during elongation of the body axis in amniote embryos”, Dev. Cell., 40: 342-353 (2017).
Mechano-responses of embryonic tissues in vitro
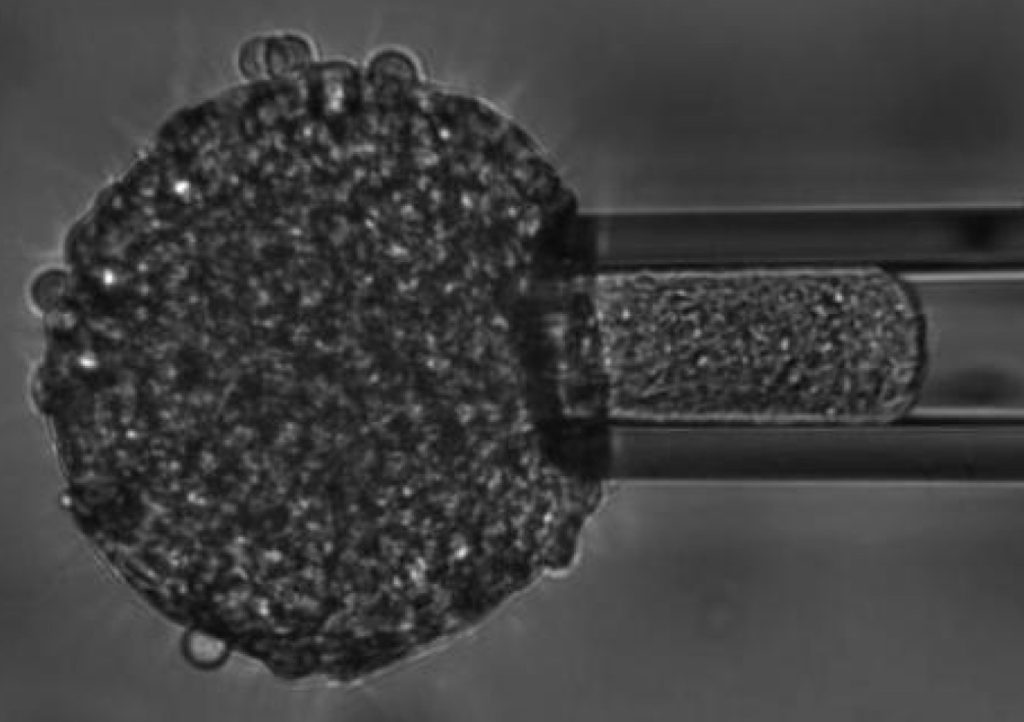
We study the mechanics of in vitro multicellular systems, like tissue explants and cellular aggregates, using techniques such as micropipette aspiration, to determine their rheology and their active responses to applied external stresses. We also investigate how such systems behave when put on adhesive substrates. Following their spreading dynamics, we can assess the role of cytoskeletal and intercellular components interfering with this process.
- K. Guevorkian, F. Brochard-Wyart, D. Gonzalez-Rodriguez, Chapter eight – “Flow dynamics of 3D multicellular systems into capillaries”, in Viscoelasticity and Collective Cell Migration, Editors: I. Pajic-Lijakovic, E. H. Barriga, Academic Press, Pages 193-223, (2021).
- K. Guevorkian, and and J.L. Maître, “Micropipette aspiration: a unique tool for exploring cell and tissue mechanics in vivo”, Methods Cell. Biol., 139:187-201 (2016).
- D. Gonzalez-Rodriguez*, K. Guevorkian*, S. Douezan*, and F. Brochard-Wyart, “Soft Matter Models of Developing Tissues and Tumors”, Science, 338 (6109): 910-917 (2012) (*equal contribution).
- K. Guevorkian*, D. Gonzalez-Rodriguez*, C. Carlier, S. Dufour, and F. Brochard-Wyart, “Mechanosensitive shivering of model tissues under controlled aspiration”, Proc. Natl. Acad. Sci. USA, 108 (33): 13387-13392 (2011) (*equal contribution).
- S. Douezan, K. Guevorkian, R. Naouar, S. Dufour, D. Cuvelier, and F. Brochard-Wyart, “Spreading dynamics and wetting transition of cellular aggregates”, Proc. Natl. Acad. Sci. USA, 108 (18): 7315-7320 (2011).
- K. Guevorkian, M. J. Colbert, M. Durth, S. Dufour, and F. Brochard-Wyart, “Aspiration of biological viscoelastic drops”, Phys. Rev. Lett., 104: 218101 (2010)
Biomimetic cells
Using a minimal biomimetic system, we explore how actin cortex might modify the mechanics of a cell’s plasma membrane. First, an actin shell is polymerized at the inner layer of a Giant Unilamellar Vesicle (GUV, aka a liposome), and then, the effect of the cortex on the mechanics of the membrane is studied. At constant force (hydrodynamic tube pulling technique), membrane nanotubes pulled from such systems show a drastic difference compared to GUVs lacking a cortex, suggesting the formation of domains, known as corals, which limit drastically the lipid area available for membrane nanotubes.
- K. Guevorkian, J. Manzi, F. Brochard-Wyart, and C. Sykes, “Mechanics of biomimetic liposomes encapsulating an actin shell”, Biophys. J., 109: 2471-2479 (2015).
- J. Lemière, K. Guevorkian, C. Campillo, C. Sykes, and T. Betz, “α-hemolysin membrane pore density measured on liposomes”, Soft Matter, 9, 3181-3187 (2013).
- M. Murrell, L. L. Pontani, K. Guevorkian, D. Cuvelier, P. Nassoy, and C. Sykes, “Spreading Dynamics of Biomimetic Actin Cortices”, Biophy. J., 100: 1400-1409 (2011).
Swimming Paramecium in magnetic fields
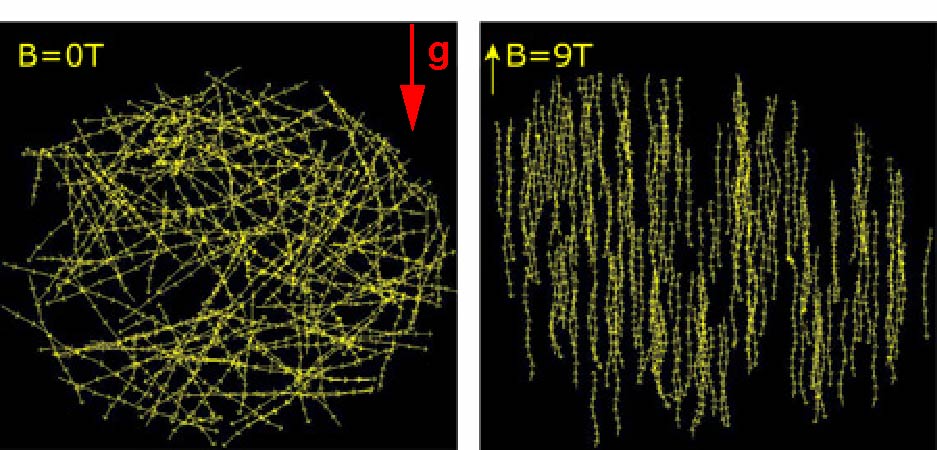
Any biological matter (70% water) can be levitated with a strong magnet, thanks to its diamagnetic properties. By exploiting this diamagnetic levitation principle, we developed a setup, using inhomogeneous intense magnetic fields, to apply pulling and pushing forces to cells. We studied the role of these simulated gravitational forces on the swimming Paramecia, and showed that they actively regulate their swimming speed in response to the strength of the gravitational force. We also showed that homogenous fields, i.e. no force, the swimming speed did not change, but the trajectories were aligned with the field direction.
- K. Guevorkian and J. M. Valles, Jr., “Swimming Paramecium in magnetically simulated enhanced, reduced and inverted gravity environments”. Proc. Natl. Acad. Sci. USA, 103(35): 13051-13056 (2006).
- K. Guevorkian and J. M. Valles, Jr., “Aligning Paramecium caudatum with static magnetic fields”, Biophys. J., 90: 3004-3011 (2006).
- K. Guevorkian and J. M. Valles, Jr., “In situ imaging of microorganisms in intense magnetic fields”, Rev. Sci. Inst., 76: 103706 (2005).
- K. Guevorkian and J. M. Valles, Jr., “Varying the effective buoyancy of cells using magnetic forces”, Appl. Phys. Lett. 84(24): 4863-4865 (2004).
- PhD Thesis manuscript.
